Received: Fri 01, Dec 2023
Accepted: Tue 26, Dec 2023
Abstract
Background and Intention: Erectile dysfunction (ED) is an underappreciated clinical condition in men. This study aims to compare the dynamic changes in the distribution of ED among male kidney transplant recipients (mKTRs) in four epochs: end-stage renal disease period (ESRDp), early post-transplant period (EPTP), pre-COVID-19, and post-COVID-19. Methods: General information was gathered through interviews, follow-ups, and medical records. The international index of erectile function questionnaire-5 was used to assess erectile function. The Mann-Whitney U test and chi-square test were used to analyze differences in ED strength. Univariate and logistic regression analyses were conducted to identify risk factors for ED. Results: The database contains 230 mKTRs. In the ESRDp, 17.0% had normal erectile function, 53.5% had mild ED, 18.3% had moderate ED, and 11.3% had severe ED. In the EPTP, the distribution was 38.2% normal, 42.6% mild, 10.8% moderate, and 8.2% severe. In the pre-COVID-19 period, it was 34.3%, 47.3%, 10.4%, and 7.8%, and in the post-COVID-19 period, it was 23.0%, 45.6%, 21.3%, and 10.0%. Overall, erectile function improved after kidney transplant (KT). However, post-COVID-19, the proportion of erectile function significantly decreased compared to EPTP and pre-COVID-19 periods. Risk factors for post-pandemic ED included degree, generalized anxiety disorder-7, kidney donor type, postoperative time, and hemoglobin concentration. Conclusion: KT improves erectile function in mKTRs within 5 years, but post-SARS-CoV-2 viral infection, ED worsens due to altered risk factors. These findings inform future research for comprehensive ED prevention and management strategies in this population.
Keywords
Erectile dysfunction, kidney transplant, SARS-CoV-2, international index of erectile function questionnaire-5
1. Introduction
Over the past 70 years, kidney transplant (KT) has emerged as the preferred and cost-effective treatment for end-stage renal disease (ESRD) when compared to long-term dialysis. Moreover, significant improvements have been made in the graft and patient survival rates post-transplantation, thanks to advanced surgical techniques and the availability of innovative immunosuppressive agents [1]. As a consequence, there has been a growing demand to enhance health-related quality of life (HRQOL) as the global number of kidney transplant recipients (KTRs) continues to rise [2]. The health of KTRs encompasses the integration of physical, mental, and social well-being, with sexual function playing a crucial role in both physical and mental health. Male erectile dysfunction (ED) represents a substantial issue on a global scale, affecting a prevalence range of 11.3 to 64 percent among sexually active men [3, 4]. ED is particularly prevalent in patients with ESRD period (ESRDp), with a prevalence exceeding 80% [5]. A significant proportion of these patients also report reduced libido and a notable decline in the frequency of sexual intercourse [6]. These issues can have significant adverse effects on immune function, cardiovascular function, sleep quality, and family dynamics.
For KTRs with ED, the clinical prognosis indicates a positive trend in ED after receiving KT [7], however, current research indicates that the immune-inflammatory response driven by SARS-CoV-2 could be just a drop in the ocean when it comes to severe clinical manifestations associated with the pulmonary and cardiovascular systems. Ultimately, there is a potential emergence of clinical diseases driven by underlying multi-organ dysfunction [8]. Additionally, many sexually active individuals are facing economic and psychological pressures, as well as health concerns driven by COVID-19, inevitably experiencing impacts in various ways [9, 10]. Emerging reports within the realm of COVID-19 complications have indicated that the initial or eventual occurrence of ED could potentially serve as an alternative marker for underlying endothelial dysfunction, carrying profound significance in the prevention of cardiovascular diseases [11]. An increasing body of research suggests intricate associations between primary organic or psychogenic ED and diseases related to SARS-CoV-2 infection [12, 13].
This study aimed to gather data on ED in male KTRs (mKTRs) at different stages, including ESRDp, EPTP, pre-COVID-19, and post-COVID-19. Does KT genuinely aid in the amelioration of ED? Are recipients who experience improvements in ED prone to relapse with the prolonged duration of KT? How does COVID-19 clinically demonstrate the adverse effects on erectile function in mKTRs? We delve into the exploration of these thought-provoking academic questions.
2. Materials and Methods
2.1. Data Collection
In this study, all mKTRs were collected from January 1, 2018, to March 1, 2022, and the specific screening process is shown in (Figure 1). This study was approved by our hospital ethics review (ethics number: PJ2023-10-47). The included indicators were age, postoperative time, deceased donor (DD) or living donor (LD), smoking (never, former smoking, current smoking), degree (elementary, junior, high school, and above), BMI (kg/cm2), location (town or rural), patient health questionnaire-9 (PHQ-9), generalized anxiety disorder-7 (GAD-7), tacrolimus plasma concentration; Biochemical indexes: total cholesterol, triglycerides, high-density lipoprotein (HDL), non-high-density lipoprotein (nHDL), very low-density lipoprotein (VLDL), low-density lipoprotein (LDL), albumin, globulin, alanine aminotransferase, glutamate aminotransferase, creatinine, eGRF).
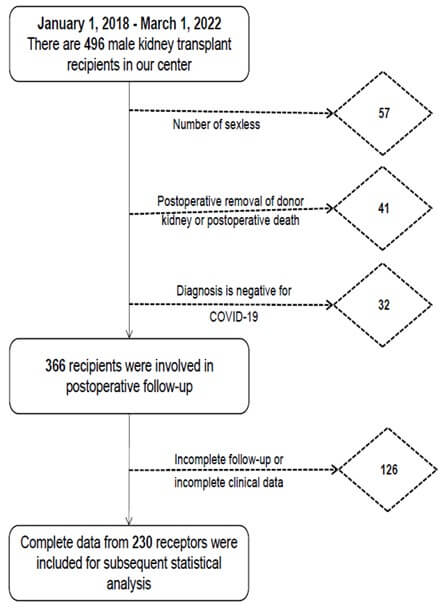
ED: Erectile Dysfunction; mKTRs: Male Kidney Transplant Recipients.
2.1.1. Blood Count
Absolute red blood cell count (RBC), absolute white blood cell count (WBC), platelet count (PLT), neutrophils, percentage neutrophils, and hemoglobin (HB).
2.1.2. Data Collection Methods
i) When mKRTs come to the outpatient follow-up, they enter a special consultation room and complete the questionnaire content with a single self-report question. If you have any questions about the content of the questionnaire, there is a professional andrologist next to answer them. Questionnaires were filled out IIEF-5, PHQ-9, and GAD-7. ii) The demographics, medical history, and laboratory data of mKTRs were obtained from the hospital's medical record system and examination system.
2.1.3. Exclusion Criteria
mKTRs in the following cases will be excluded. i) No stable sex life. ii) Patients who die or have allograft removed after KT. iii) Diagnosis is negative for SARS-CoV-2. iv) Those who have not completed the follow-up visit completely or have lost clinical data.
2.2. Definition
2.2.1. ED
The persistent inability to achieve and maintain an erection sufficient for satisfying sexual activity [14].
2.2.2. SARS-CoV-2 Testing Criteria
The nucleic acid amplification testing method is used to detect the presence of SARS-CoV-2 RNA in respiratory specimens (nasopharyngeal swabs, oropharyngeal swabs, sputum, tracheal aspirates), or other specimens. Fluorescent quantitative PCR is currently the most commonly used method for SARS-CoV-2 RNA detection.
2.2.3. ESRDp
From the day your doctor diagnoses ESRD until the day you have an allogeneic kidney transplant.
2.2.4. EPTP
The period is counted from the day of discharge and is limited to 6 months after discharge.
2.2.5. Pre-COVID-19
The time is counted from the last day of the EPTP until the test result is positive for SARS-CoV-2.
2.2.6. Post-COVID-19
It starts with a negative SARS-CoV-2 test and continues for a duration of 3 months, during which subsequent retests also yield negative results.
2.2.7. IIEF-5, PHQ-9, GAD-7
The diagnostic criteria for ED are in the form of IIEF-5 scoring. A maximum score of 25, 0-7 is severe, 8-11 is moderate, 12-21 is mild, and above 22 is normal. The degree of depression is evaluated according to the score on the PHQ-9 scale. A maximum score of 27, 0-4 is no depression, 5-9 is mild, 10-14 is moderate, and 15 or more is severe. Anxiety symptoms are evaluated on a GAD-7 scale. A maximum score of 21, 0-4 is no anxiety symptoms, 5-9 is mild, 10-14 is moderate, and above 15 is severe.
2.3. Statistical Analysis
Continuous variables are shown as mean (SD) for normally distributed variables or median [interquartile range (IQR)] for skewed variables, and categorical variables as numbers (%). To assess the overall trend and distribution differences of IIEF-5 scores among the 230 recipients in the database across four time periods, the Kruskal-Wallis test using rank sums is employed. Group comparisons are conducted using the Wilcoxon signed-rank test or the Mann-Whitney U test. The comparison of the prevalence of ED between groups was carried out using the Bonferroni method of the chi-square test. In the post-pandemic era, univariate analysis and binary logistic regression analysis methods were used to explore the risk factors leading to ED, and the regression coefficients, p-values, and confidence intervals for each independent variable were obtained. Data processing and charting use R code version 4.2.0, SPSS version 26.0, and GraphPad Prism version 8.0.1 software. P<0.05 is shown to be statistically significant, and p'<0.008, adjusted in Bonferroni's method, is statistically significant.
3. Results
The dataset of 230 mKTRs included was grouped according to four periods, and each group was compared with two branches, normal and ED, as detailed in (Table 1).
Based on the IIEF-5 score, (Table 2) shows the dynamic trend of mKTRs over four periods of ED. To compare whether there is a difference in the overall distribution of ED in the four periods, we plot a box plot for visual comparison, as shown in (Figure 2), it can be seen that there are significant statistical differences in the distribution of ED in the four periods, except for ESRDp and post-COVID-19. To compare whether erectile recovery rates improved over the four periods, we plotted stacked histograms to visually compare the mKTRs population, as shown in (Figure 3A), which showed that the normal group had a significant increase in EPTP, and by pre-COVID-19, there was no statistically different distribution between the normal group and the EPTP. This indicates that the good trend of KT-improved ED has not changed in the short term of 5 years. In the comparison of post-COVID-19 with pre-COVID-19, the proportion of normal groups is further reduced. For mKTRs for mild and moderate ED, we also plotted histogram stacked plots for four periods of the population to illustrate the statistical results, as shown in (Figure 3B). It can be seen that the proportion of the 'mild+moderate' group has decreased significantly in KT. This group increased significantly after suffering from the COVID-19 pandemic.
TABLE
1: Baseline
characteristics of ED recipients compared to normal receptors: demographic and
clinical data.
|
230, Mean+SD / N (%) |
||||||||
|
ESRD |
EPTP |
pre-COVID19 |
post-COVID19 |
|||||
|
Characteristics |
Normal (39,17.0%) |
ED (191,83.0%) |
Normal (88,38.3%) |
ED (142,61.7%) |
Normal (79,34.3%) |
ED (151,65.7%) |
Normal (53,23.0%) |
ED (177,77.0%) |
|
Age (year) |
37.8 ± 8.7 |
40.7 ± 9.9 |
39.2 ±
9.6 |
40.9 ±
9.8 |
39.6 ± 9.7 |
40.6 ± 9.8 |
39.4 ±
9.9 |
40.5 ±
9.7 |
|
Postoperative
time (month) |
31.6 ± 15.0 |
33.4 ± 15.3 |
32.8 ±
14.1 |
33.3 ±
16.0 |
33.6 ± 15.0 |
32.8 ± 15.5 |
36.0 ±
15.0 |
32.2 ±
15.3 |
|
BMI
(kg/cm2) |
22.5 ± 3.3 |
22.6 ± 3.7 |
22.9 ±
3.9 |
22.4 ±
3.4 |
22.9 ± 4.1 |
22.5 ± 3.4 |
22.5 ±
2.8 |
22.7 ± 3.8 |
|
Total
protein (g/L) |
70.0 ± 9.7 |
71.0 ± 10.2 |
65.1 ±
7.7 |
62.5 ±
7.1 |
69.8 ± 5.2 |
69.8 ± 4.9 |
70.4 ±
4.3 |
69.3 ±
6.7 |
|
Albumin
(g/L) |
42.5 ± 6.1 |
43.0 ± 6.8 |
40.3 ±
6.1 |
38.8 ±
5.6 |
45.0 ± 3.6 |
45.0 ± 4.2 |
46.9 ±
2.6 |
46.6 ±
3.7 |
|
Globulin
(g/L) |
27.5 ± 4.8 |
28.2 ± 5.5 |
24.8 ±
4.1 |
23.7 ±
3.7 |
24.7 ± 4.0 |
24.7 ± 3.9 |
23.5 ±
3.3 |
23.3 ±
4.0 |
|
Alanine
aminotransferase (u/L) |
21.8 ± 16.7 |
19.4 ± 11.5 |
34.4 ±
36.7 |
30.1 ±
31.4 |
24.0 ± 15.2 |
26.4 ± 43.2 |
22.3 ±
23.3 |
19.6 ±
16.0 |
|
Glutamate
aminotransferase (u/L) |
18.7 ± 8.6 |
18.4 ± 7.7 |
21.5 ±
13.8 |
21.3 ±
16.6 |
20.4 ± 7.6 |
23.9 ± 42.0 |
16.8 ±
12.5 |
16.4 ±
9.1 |
|
Creatinine
(umol/L) |
1003.5 ± 361.7 |
1062.2 ± 316.0 |
140.7 ±
45.2 |
145.6 ±
46.6 |
177.2 ± 126.8 |
201.8 ± 185.1 |
151.2 ±
52.8 |
154.5 ±
59.0 |
|
RBC
(*1012/L) |
3.4 ± 0.7 |
3.5 ± 0.9 |
6.4 ±
20.0 |
4.2 ± 11.3 |
4.5 ± 0.8 |
4.3 ± 0.9 |
4.4 ±
0.7 |
4.4 ±
0.8 |
|
WBC
(*109/L) |
7.0 ± 3.0 |
6.9 ± 2.3 |
8.2 ±
3.1 |
8.8 ±
9.2 |
7.0 ± 2.5 |
7.7 ± 8.9 |
9.1 ±
15.6 |
8.4 ±
10.5 |
|
PLT
(*109/L) |
180.6 ± 57.9 |
179.5 ± 61.0 |
202.2 ±
69.0 |
197.7 ±
71.2 |
183.5 ± 60.2 |
183.1 ± 63.9 |
194.0 ±
63.4 |
183.6 ±
66.4 |
|
HB
(g/L) |
103.6 ± 20.2 |
106.7 ± 24.8 |
98.2 ±
26.6 |
96.9 ±
23.3 |
132.3 ± 24.6 |
126.5 ± 25.2 |
134.5 ±
19.7 |
128.5 ±
29.2 |
|
Neutrophils
(*109/L) |
5.0 ± 3.0 |
4.8 ± 2.4 |
6.1 ±
2.8 |
9.4 ±
41.4 |
4.6 ± 1.9 |
4.4 ± 1.7 |
4.4 ±
1.6 |
5.3 ±
6.2 |
|
Percent
neutrophils (%) |
68.6 ± 11.5 |
67.0 ± 12.0 |
72.8 ±
9.6 |
72.0 ±
11.4 |
64.8 ± 9.0 |
63.1 ± 10.4 |
61.3 ±
11.6 |
67.0 ±
43.8 |
|
Tacrolimus
concentration (ng/ml) |
- |
- |
11.5 ±
5.3 |
13.0 ±
12.6 |
6.1 ± 2.8 |
5.9 ± 2.0 |
6.1 ±
2.3 |
6.1 ±
2.3 |
|
Total
cholesterol (mmol/L) |
- |
- |
- |
- |
5.5 ± 6.8 |
5.0 ± 5.0 |
4.9 ±
1.2 |
4.7 ±
1.1 |
|
Triglycerides
(mmol/L) |
- |
- |
- |
- |
1.9 ± 1.2 |
2.1 ± 1.3 |
1.8 ±
1.1 |
1.9 ±
1.1 |
|
HDL-C
(mmol/L) |
- |
- |
- |
- |
1.4 ± 0.4 |
1.3 ± 0.4 |
1.4 ±
0.4 |
1.3 ±
0.3 |
|
n-HDL
(mmol/L) |
- |
- |
- |
- |
3.4 ± 0.9 |
3.3 ± 1.0 |
3.5 ±
1.2 |
3.4 ±
1.1 |
|
VLDL
(mmol/L) |
- |
- |
- |
- |
0.7 ± 0.5 |
0.8 ± 0.5 |
0.6 ±
0.4 |
0.7 ±
0.4 |
|
LDL
(mmol/L) |
- |
- |
- |
- |
2.7 ± 0.8 |
2.6 ± 0.9 |
3.1 ±
1.1 |
3.0 ±
1.0 |
|
DD or
LD |
||||||||
|
DD |
13 (33.3%) |
103 (53.9%) |
41
(46.6%) |
75
(52.8%) |
35 (44.3%) |
81 (53.6%) |
21
(39.6%) |
95
(53.7%) |
|
LD |
26 (66.7%) |
88 (46.1%) |
47
(53.4%) |
67
(47.2%) |
44 (55.7%) |
70 (46.4%) |
32
(60.4%) |
82
(46.3%) |
|
Smoking
status |
||||||||
|
never |
26 (66.7%) |
138 (72.3%) |
54
(61.4%) |
110
(77.5%) |
50 (63.3%) |
114 (75.5%) |
37
(69.8%) |
127
(71.8%) |
|
former |
7 (17.9%) |
44 (23.0%) |
24
(27.3%) |
27
(19.0%) |
22 (27.8%) |
29 (19.2%) |
10
(18.9%) |
41 (23.2%) |
|
current |
6 (15.4%) |
9 (4.7%) |
10
(11.4%) |
5
(3.5%) |
7 (8.9%) |
8 (5.3%) |
6
(11.3%) |
9
(5.1%) |
|
Degree |
||||||||
|
primary
school |
1 (2.6%) |
27 (14.1%) |
6
(6.8%) |
22
(15.5%) |
6 (7.6%) |
22 (14.6%) |
2
(3.8%) |
26
(14.7%) |
|
middle
school |
16 (41.0%) |
86 (45.0%) |
37 (42.0%) |
65
(45.8%) |
34 (43.0%) |
68 (45.0%) |
22
(41.5%) |
80
(45.2%) |
|
high
school |
6 (15.4%) |
35 (18.3%) |
11
(12.5%) |
30
(21.1%) |
10 (12.7%) |
31 (20.5%) |
10
(18.9%) |
31
(17.5%) |
|
>high school |
16 (41.0%) |
43 (22.5%) |
34
(38.6%) |
25
(17.6%) |
29 (36.7%) |
30 (19.9%) |
19 (35.8%) |
40
(22.6%) |
|
Address |
||||||||
|
town |
18 (46.2%) |
87 (45.5%) |
44
(50.0%) |
61
(43.0%) |
39 (49.4%) |
66 (43.7%) |
27
(50.9%) |
78
(44.1%) |
|
rural |
21 (53.8%) |
104 (54.5%) |
44
(50.0%) |
81
(57.0%) |
40 (50.6%) |
85 (56.3%) |
26
(49.1%) |
99
(55.9%) |
|
Grade_PHQ9 |
||||||||
|
Normal |
14 (35.9%) |
44 (23.0%) |
54
(61.4%) |
79
(55.6%) |
52 (65.8%) |
86 (57.0%) |
18
(34.0%) |
51
(28.8%) |
|
mild |
16 (41.0%) |
52 (27.2%) |
20
(22.7%) |
44
(31.0%) |
13 (16.5%) |
33 (21.9%) |
15
(28.3%) |
45
(25.4%) |
|
moderate |
3 (7.7%) |
38 (19.9%) |
11
(12.5%) |
15
(10.6%) |
14 (17.7%) |
26 (17.2%) |
16
(30.2%) |
46
(26.0%) |
|
severe |
6 (15.4%) |
57 (29.8%) |
3
(3.4%) |
4
(2.8%) |
0 (0.0%) |
6 (4.0%) |
4
(7.5%) |
35
(19.8%) |
|
Grade_GAD7 |
||||||||
|
Normal |
19 (48.7%) |
79 (41.4%) |
65
(73.9%) |
98
(69.0%) |
59 (74.7%) |
111 (73.5%) |
33
(62.3%) |
71
(40.1%) |
|
mild |
11 (28.2%) |
64 (33.5%) |
19
(21.6%) |
31
(21.8%) |
17 (21.5%) |
32 (21.2%) |
12
(22.6%) |
73
(41.2%) |
|
moderate |
7 (17.9%) |
22 (11.5%) |
3
(3.4%) |
11
(7.7%) |
1 (1.3%) |
5 (3.3%) |
6
(11.3%) |
23
(13.0%) |
|
severe |
2 (5.1%) |
26 (13.6%) |
1
(1.1%) |
2
(1.4%) |
2 (2.5%) |
3 (2.0% |
2
(3.8%) |
10 (5.6%) |
ED: Erectile Dysfunction;
EPTP: Early Post-Transplant Period; HB: Hemoglobin; HDL: High-Density
Lipoprotein; HDL-C: HDL Cholesterol; VLDL: Very Low-Density Lipoprotein; LDL: Low-Density
Lipoprotein; DD: Deceased Donor; LD: Living Donor.
TABLE
2: ED disease
profile overview.
|
|
Normal |
Mild |
Moderate |
Severe |
Total |
|
ESRD |
39 |
123 |
42 |
26 |
230 |
|
EPTP |
88 |
98 |
25 |
19 |
230 |
|
pre-COVID19 |
79 |
109 |
24 |
18 |
230 |
|
post-COVID19 |
53 |
105 |
49 |
23 |
230 |
|
Total |
259 |
435 |
140 |
86 |
920 |
ED: Erectile Dysfunction;
EPTP: Early Post-Transplant Period.
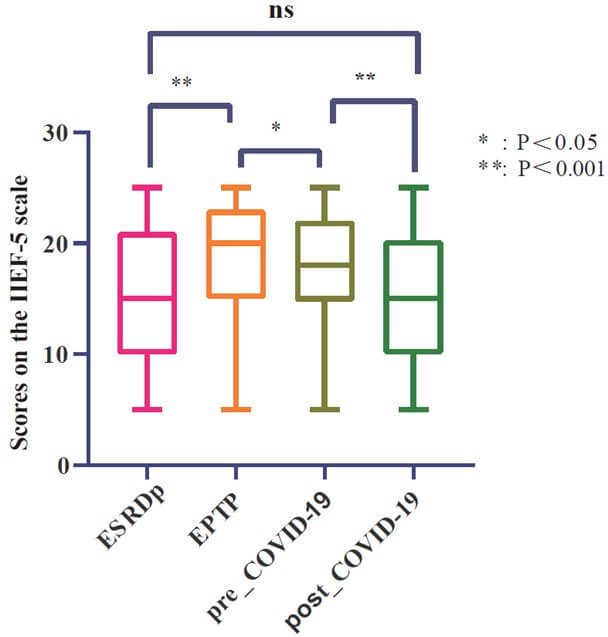
EPTP: Early Post-Transplant Period; IIEF-5: International Index of Erectile Function Questionnaire-5.
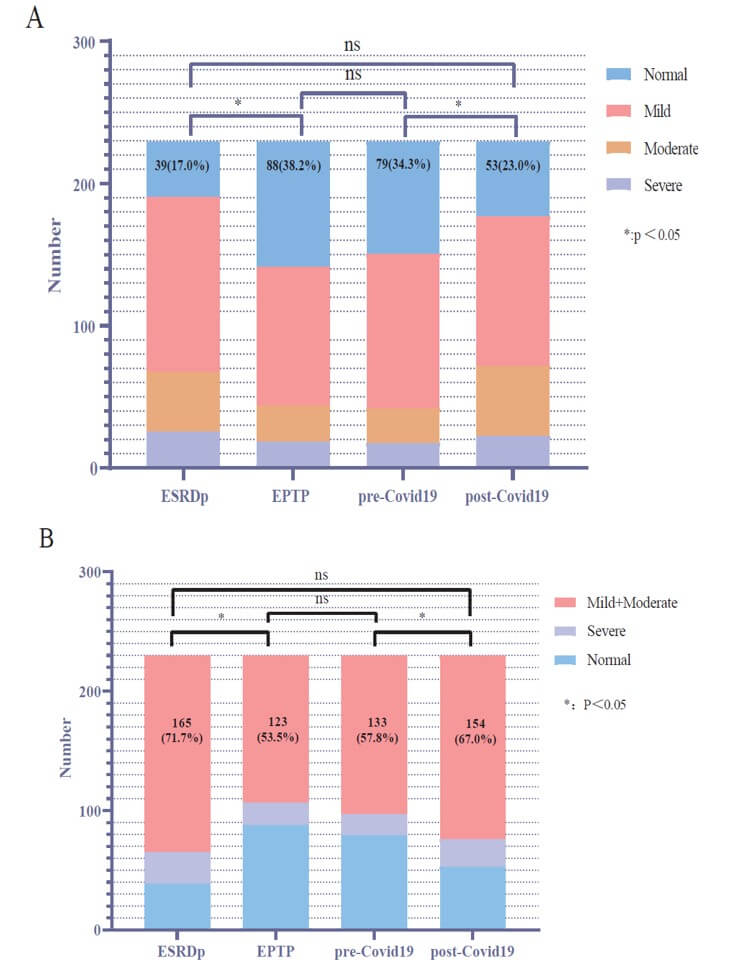
EPTP: Early Post-Transplant Period.
'*' and 'ns' represent the statistical results of the 'Normal' group (Box plot A) or 'Mild+Moderate' group (Box plot B) over four time periods.
In the post-pandemic era, we performed internal analysis for risk factors that may cause mKTRs to fall into ED, and the results are shown in (Table 3 & Figure 4). The results of univariate analysis (Table 3) showed that risk factors for ED included degree, Grade_GAD7, Grade_PHQ9, DD or LD, age (years), smoking status, postoperative time (month), and HB (g/L). According to the odds Ratio (OR) observations, the risk of ED in mKTRs decreases gradually with higher levels of education and HB content. However, it is positively correlated with anxiety, depression, kidney donation from deceased donors, age, and smoking. The multivariate logistic regression analysis in (Figure 4) showed that five factors, including degree, HB (g/L), postoperative time (month), Grade_GAD7, and DD or LD, were strongly associated with the occurrence of ED.
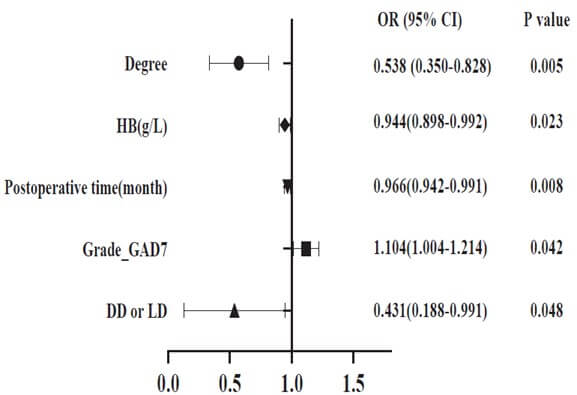
ED: Erectile Dysfunction; mKTRs: Male Kidney Transplant Recipients; DD: Deceased Donor; LD: Living Donor; HB: Hemoglobin.
TABLE
3: Univariate
analysis of risk factors associated with ED in the post-pandemic era.
|
Characteristics |
OR
(95%CI) |
P
value |
|
Degree |
0.678
(0.497-0.926) |
0.014 |
|
Grade_GAD7 |
3.387
(5.011-1.169) |
0.024 |
|
Grade_PHQ9 |
1.449
(2.991-1.110) |
0.028 |
|
DD
or LD |
2.566
(4.303-1.058) |
0.034 |
|
Age
(year) |
2.412
(4.980-1.045) |
0.036 |
|
|
Smoking
status |
1.805
(3.493-1.314) |
0.042 |
|
Postoperative
time (month) |
1.984
(3.965-1.004) |
0.043 |
|
HB
(g/L) |
0.891
(0.779-1.006) |
0.045 |
|
HDL-C
(mmol/L) |
0.407
(0.166-0.999) |
0.05 |
|
Total protein
(g/L) |
0.964
(0.904-1.028) |
0.26 |
|
PLT
(*10^9/L) |
0.998
(0.993-1.002) |
0.312 |
|
Total
cholesterol (mmol/L) |
0.868
(0.661-1.138) |
0.312 |
|
Glutamate
aminotransferase (u/L) |
0.992
(0.977-1.008) |
0.338 |
|
Neutrophils
(*10^9/L) |
1.095
(0.905-1.326) |
0.349 |
|
Address |
1.318
(0.713-2.438) |
0.379 |
|
Percent
neutrophils (%) |
1.011
(0.985-1.038) |
0.402 |
|
LDL
(mmol/L) |
0.918
(0.678-1.242) |
0.577 |
|
WBC
(*10^9/L) |
0.995
(0.972-1.019) |
0.68 |
|
Albumin
(g/L) |
0.982
(0.899-1.072) |
0.682 |
|
Triglycerides
(mmol/L) |
1.061 (0.798-1.410) |
0.684 |
|
Creatinine
(umol/L) |
1.001
(0.996-1.007) |
0.714 |
|
Alanine
aminotransferase (u/L) |
0.995
(0.966-1.025) |
0.754 |
|
Globulin
(g/L) |
0.988
(0.912-1.070) |
0.761 |
|
n-HDL
(mmol/L) |
0.960
(0.726-1.269) |
0.773 |
|
BMI
(kg/cm2) |
1.013
(0.928-1.105) |
0.777 |
|
Tacrolimus
concentration (ng/ml) |
1.004
(0.878-1.148) |
0.954 |
|
RBC
(*10^12/L) |
1.000
(0.684-1.461) |
0.999 |
ED: Erectile Dysfunction;
HDL: High-Density Lipoprotein; HDL-C: HDL Cholesterol; VLDL: Very Low-Density
Lipoprotein; LDL: Low-Density Lipoprotein; DD: Deceased Donor; LD: Living
Donor.
4. Discussion
In the pathogenesis of ESRD with high incidence ED, it is currently believed to be caused by multiple factors. Various factors contribute to its development, including abnormalities in the hypothalamic-pituitary-gonadal axis, disturbances in the autonomic nervous system, peripheral neuropathy, endothelial dysfunction, anemia, secondary hyperparathyroidism, medication effects, and psychological factors like stress and depression. These factors collectively play a role in the occurrence of ED, albeit to varying degrees [15]. Immunosuppressants and antihypertensive medications are involved in the occurrence of ED in mKTRs. Specifically, calcineurin inhibitors such as cyclosporine and tacrolimus, mTOR inhibitors, and corticosteroids may impact endothelial function and/or testicular function/structure [16]. This supports our research finding that kidney transplant therapy in ESRD patients lowers the rate of ED, though it still exceeds that of the general population.
From an epidemiological perspective, as KTRs live longer than ever, it is crucial to prioritize their HRQOL. Among them, the incidence of ED in mKTRs was generally between 54% and 66% [17, 18], and our center was 61.8%, which improved the ED status of mKTRs by 21.2% compared with 83% during the ESRDp. These findings further support previous report which suggest that KT significantly improves ED [19]. However, the situation is not entirely optimistic. During the questionnaire collection process, it was discovered that many mKTRs with severe ED reported that their ED persisted even after undergoing KT, with little improvement observed. In the post-pandemic era, the reported trends of ED among mKTRs remain unknown. The initial findings from our center indicate a prevalence rate of 77%. When compared to the ESRDp group, there were no significant statistical differences observed in the distribution of the average International Index of IIEF-5 scores, as illustrated in (Figure 2). Additionally, there were no statistically significant differences observed in the proportion of individuals with normal erectile function when compared to the ESRDp group, as shown in (Figure 3A). Has the impact of the COVID-19 pandemic on mKTRs in terms of erectile function offset the improvement in KT? This also requires multi-center further verification.
In the treatment of ED, various approaches are available, including medication and non-pharmacological interventions. Psychological therapy is another option. Extracorporeal low-intensity shockwave therapy is an external treatment modality. For specific cases, vascular surgery may be considered, and testosterone replacement therapy is also an option [20]. It has been proven that mild and moderate ED show better treatment outcomes with medication or other methods [21, 22], so we focused on this demographic. According to the stacked bar chart in (Figure 3B), the 'mild+moderate' group represents the majority of cases of ED, and its proportion varies inversely with the number of individuals in the normal group across the four time periods. In the post-pandemic era, there is no statistically significant difference in the number of individuals with ‘mild+moderate’ ED compared to the ESRDp group. This suggests that in the post-pandemic era, there is an increasing incidence of ED among mKTRs, and a significant portion of the ED population may have transitioned from the normal group. To enhance the HRQOL for mKTRs, andrologists, and kidney transplant specialists should allocate more energy and time towards addressing the treatment of mild or moderate ED.
Understanding the risk factors associated with ED is a critical theoretical basis in the dimension of preventing recurrence or worsening of ED. The risk factors influencing ED encompass both organic factors and psychogenic and relationship factors. Previous literature has reported that lifestyle factors such as smoking, alcohol consumption, obesity, and excessive intake of red meat can contribute to the occurrence of ED [23, 24]. Our study also found a strong correlation between epidemiological data and ED, such as the degree. Education is inversely correlated with ED, possibly because people with low levels of education pay less attention to health care, quality of life, and sexuality [25]. mKTRs who received grafts from LD and had higher education levels were found to have a lower risk of developing ED. Previous literature has also reported that LD compared to DD showed better quality of life, reduced fatigue, and improved social participation [26], which may also reflect positively on the occurrence of ED.
Analyzing from the perspective of psychogenic and relationship factors, the widespread fear of mKTRs and the global population towards COVID-19, along with the uncertainty of the future, financial and economic losses, and reduced social support during lockdown, have exacerbated psychological distress, depression, and anxiety among individuals in the population [27]. Both of these conditions are closely associated with the occurrence and development of ED [28]. Previous research had primarily focused on the quantitative impact of depression on ED. However, in the post-pandemic era, this focal point may shift. Given the global spread of COVID-19, sensitive populations like KTRs may be more significantly affected by anxiety. Furthermore, our center has confirmed this observation by analyzing the impact of PHQ-9 and GAD-7 on ED. As seen in (Table 3 & Figure 4), the severity of anxiety is positively correlated with the incidence of ED in mKTRs, while the correlation between depression and ED is not significant.
Naturally, there are several limitations to this study. Firstly, as this is the first study to investigate the data regarding ED in mKTRs in the post-pandemic era, the results need to be validated and supplemented by multicenter studies. Secondly, the scoring used to diagnose ED relied on a single self-report question. Due to the wide period covered by the four scenarios, there may be biases in patients' retrospective reports of erectile function in the previous three scenarios. Lastly, testosterone’s impact on ED and testosterone replacement therapy for treating ED are hot topics [29]. This study did not collect data on the hormonal indicators in KTRs, and further exploration is needed to investigate the relationship between ED and these factors.
5. Conclusion
This study has revealed the dynamic trends in the distribution of ED among mKTRs during four crucial periods. KT can improve erectile function in mKTRs and appears to be effective within 5 years. Additionally, it highlights the worsening of erectile function in mKTRs following the impact of COVID-19. These findings provide a foundation for further research, aiming to develop comprehensive strategies for preventing and managing ED in this patient population.
Acknowledgments
The authors are grateful for the invaluable support and useful discussions with other members of the urological department.
Conflicts of Interest
None.
Funding
This work was supported by the National Natural Science Foundation of China (82070724).
Author Contributions
All authors substantially contributed to the conception, design, and planning of the study. JS.P., ZM.Z., WB.W., ZY.H., and GY.L. substantially contributed to the drafting of the manuscript. All authors substantially contributed to critically reviewing or revising the manuscript for important intellectual content.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.
REFERENCES
[1] Maarten Coemans, Caner Süsal, Bernd
Döhler, et al. “Analyses of the short- and long-term graft survival after
kidney transplantation in Europe between
1986 and 2015.” Kidney Int, vol. 94,
no. 5, pp. 964-973,
2018. View at: Publisher
Site | PubMed
[2] Denise M J Veltkamp, Yiman Wang,
Yvette Meuleman, et al. “Age and gender differences in symptom experience and
health-related quality of life in kidney
transplant recipients: a cross-sectional study.” Nephrol Dial Transplant, vol. 38, no. 7, pp. 1707-1718, 2023. View at: Publisher Site | PubMed
[3] Jerel P Calzo, S Bryn Austin,
Brittany M Charlton, et al. “Erectile Dysfunction in a Sample of Sexually
Active Young Adult Men from a U.S.
Cohort: Demographic, Metabolic and Mental Health Correlates.” J Urol, vol. 205, no. 2, pp. 539-544, 2021. View at: Publisher Site | PubMed
[4] C B Pinnock, A M Stapleton, V R
Marshall “Erectile dysfunction in the community: a prevalence study.” Med J Aust, vol. 171, no. 7, pp. 353-357, 1999. View at: Publisher
Site | PubMed
[5] Nikolaos Pyrgidis, Ioannis
Mykoniatis, Meletios P Nigdelis, et al. “Prevalence of Erectile Dysfunction in
Patients With End-Stage Renal Disease: A
Systematic Review and Meta-Analysis.” J Sex Med, vol. 18, no. 1, pp. 113-120, 2021. View at: Publisher Site | PubMed
[6] Pedro Iglesias, Juan J Carrero, Juan
J Díez “Gonadal dysfunction in men with chronic kidney disease: clinical
features, prognostic implications and therapeutic options.” J Nephrol, vol. 25, no. 1, pp. 31-42, 2012. View at: Publisher Site | PubMed
[7] Hany Mohamed El Hennawy, Omar Safar,
Abdullah S Al Faifi et al. “Does Kidney Transplantation Help Young Patients on
Dialysis With Erectile Dysfunction? A
Single-center Study.” Urology, vol.
169, pp. 120-124,
2022. View at: Publisher
Site | PubMed
[8] Toshiaki Iba, Jean Marie Connors,
Jerrold H Levy “The coagulopathy, endotheliopathy, and vasculitis of COVID-19.”
Inflamm Res, vol. 69, no. 12, pp.
1181-1189, 2020.
View at: Publisher
Site | PubMed
[9] Issam Nessaibia, Raffaello Sagese,
Leo Atwood, et al. “The way COVID-19 transforms our sexual lives.” Int J Impot Res, vol. 34, no. 2, pp.
117-119, 2022. View
at: Publisher
Site | PubMed
[10] Dong Fang, Jing Peng, Shujie Liao et
al. “An Online Questionnaire Survey on the Sexual Life and Sexual Function of
Chinese Adult Men During the Coronavirus
Disease 2019 Epidemic.” Sex Med, vol.
9, no. 1, pp. 100293,
2021. View at: Publisher
Site | PubMed
[11] Andrea Sansone, Daniele Mollaioli, Erika
Limoncin, et al. “The Sexual Long COVID (SLC): Erectile Dysfunction as a
Biomarker of Systemic Complications for
COVID-19 Long Haulers.” Sex Med Rev,
vol. 10, no. 2, pp. 271-285, 2022. View at: Publisher
Site | PubMed
[12] Andrea Sansone, Daniele Mollaioli,
Giacomo Ciocca, et al. “"Mask up to keep it up": Preliminary evidence
of the association between erectile
dysfunction and COVID-19.” Andrology,
vol. 9, no. 4, pp. 1053-1059, 2021. View at: Publisher
Site | PubMed
[13] Ahmed M Bakr, Ahmed I El-Sakka
“Erectile dysfunction among patients and health care providers during COVID-19
pandemic: A systematic review.” Int J
Impot Res, vol. 34, no. 2, pp. 145-151, 2022. View at: Publisher Site | PubMed
[14] “NIH Consensus Conference. Impotence.
NIH Consensus Development Panel on
Impotence.” JAMA, vol. 270,
no. 1, pp. 83-90,
1993. View at: PubMed
[15] Biff F Palmer, Deborah J Clegg
“Gonadal dysfunction in chronic kidney disease.” Rev Endocr Metab Disord, vol. 18, no. 1, 117-130, 2017. View at: Publisher Site | PubMed
[16] Li Chen Teng, Chang Xi Wang, Lizhong
Chen “Improved erectile function and sex hormone profiles in male Chinese
recipients of kidney transplantation.” Clin Transplant, vol. 25, no. 2, pp.
265-269, 2011. View
at: Publisher
Site | PubMed
[17] B Malavaud, L Rostaing, P Rischmann,
et al. “High prevalence of erectile dysfunction after renal transplantation.” Transplantation, vol. 69, 10, pp.
2121-2124, 2000.
View at: Publisher
Site | PubMed
[18] Hamoud H Al Khallaf “Analysis of
sexual functions in male nondiabetic hemodialysis patients and renal transplant recipients.” Transpl Int, vol. 23, no. 2, pp. 176-181, 2010. View at: Publisher Site | PubMed
[19] Adelina Miron, Anca-Elena Stefan,
Ionuţ Nistor, et al. “The impact of renal transplantation
on sexual function in males with end-stage
kidney disease: a systematic review and meta-analysis.” Int Urol Nephrol, vol. 55, no. 3, pp.
563-577, 2023.
View at: Publisher
Site | PubMed
[20] Konstantinos Hatzimouratidis, Andrea
Salonia, Ganesan Adaikan, et al. “Pharmacotherapy for Erectile Dysfunction:
Recommendations From the Fourth
International Consultation for Sexual Medicine (ICSM 2015).” J Sex Med, vol. 13, no. 4, pp. 465-488, 2016. View at: Publisher Site | PubMed
[21] I Eardley, R Morgan, W Dinsmore, et
al. “Efficacy and safety of sildenafil citrate in the treatment of men with
mild to moderate erectile dysfunction.” Br J Psychiatry, vol. 178, pp. 325-330, 2001. View at: Publisher Site | PubMed
[22] Carolina Sandoval-Salinas, José P
Saffon, Juan M Martínez, et al. “Are Radial Pressure Waves Effective for the
Treatment of Moderate or Mild to
Moderate Erectile Dysfunction? A Randomized Sham Therapy Controlled Clinical
Trial.” J Sex Med, vol. 19, no. 5,
pp. 738-744, 2022.
View at: Publisher
Site | PubMed
[23] Shiyi Cao, Yong Gan, Xiaoxin Dong, et
al. “Association of quantity and duration of smoking with erectile dysfunction:
a dose-response meta-analysis.” J Sex Med,
vol. 11, no. 10, pp. 2376-2384, 2014. View at: Publisher
Site | PubMed
[24] Giovanni Corona, Giulia Rastrelli,
Sandra Filippi, et al. “Erectile dysfunction and central obesity: an Italian
perspective.” Asian J Androl, vol.
16, no. 4, pp. 581-591, 2014. View at: Publisher
Site | PubMed
[25] Ye Tian, Zheng-guo Ji, Ya-wang Tang,
et al. “Prevalence and influential factors of erectile dysfunction in male
renal transplant recipients: a multiple
center survey.” Chin Med J (Engl),
vol. 121, no. 9, pp. 795-799, 2008. View at: PubMed
[26] Ingrid B de Groot, J Iraida E Veen,
Paul J M van der Boog, et al. “Difference in quality of life, fatigue and
societal participation between living
and deceased donor kidney transplant recipients.” Clin Transplant, vol. 27, no. 4, pp. E415-E423, 2013. View at: Publisher Site | PubMed
[27] Emmanuele A Jannini, Marita P McCabe,
Andrea Salonia, et al. “Organic vs. psychogenic? The Manichean diagnosis in
sexual medicine.” J Sex Med, vol. 7,
no. 5, pp. 1726-1733,
2010. View at: Publisher
Site | PubMed
[28] Bobby B Najari, James A Kashanian “Erectile Dysfunction.” JAMA, vol. 316, no. 17, pp. 1838, 2016. View at: Publisher Site | PubMed
[29] Andrea M Isidori, Jacques Buvat, Giovanni Corona, et al. “A critical analysis of the role of testosterone in erectile function: from pathophysiology to treatment-a systematic review.” Eur Urol, vol. 65, no. 1, pp. 99-112, 2014. View at: Publisher Site | PubMed
