Received: Sat 26, Aug 2023
Accepted: Thu 14, Sep 2023
Abstract
Background and Objectives: Esophageal cancer carries a high rate of recurrence after resection. This study investigates post-recurrence survival and risk factors for recurrence after esophagectomy. Methods: Patients who underwent esophagectomy for carcinoma of the esophagus between 2007-2019 were included in a retrospective analysis. Survival between groups was compared with the log rank test. Treatment and disease related factors were tested for association with recurrence. Results: 226 patients were included with adenocarcinoma (n=206, 91.2%) and squamous cell carcinoma (n=20, 8.8%). Majority underwent neoadjuvant treatment (n=164, 72.6%). During a median 3.5 year follow up, recurrence occurred in 76 (33.6%) patients, 34 locoregional (44.7%), 27 distant (35.5%) and 15 mixed (19.7%). Median overall and disease-free survival were 3.6 and 2.4 years, respectively. No difference in post-recurrence survival was noted between locoregional, distant or mixed recurrence (7.8 vs 5.5 vs 5.3 months, respectively, p=0.59). Margin positivity, lymph node ratio (LNR) >0.2 and high tumor grade correlated with recurrence (OR 5.4, 95% CI 1.8 - 14.8, OR 2.4, 95% 1.2 - 4.8 and OR 1.8, 95% CI 0.9 - 3.4, respectively) on univariate analysis. Only positive nodal status correlated with recurrence on multivariate analysis (OR 3.69, 95% CI: 1.44 - 9.47). Conclusion: Location of recurrence did not affect survival in our cohort. Adjuvant systemic therapy should be considered in patients with higher risk of recurrence.
Keywords
Esophagectomy, esophageal neoplasms, adenocarcinoma, risk factors, retrospective studies
1. Introduction
Esophageal carcinoma has traditionally carried a high recurrence rate after surgery which has led to overall poor prognosis [1]. Five-year survival for locally advanced disease (≥T2, any N) rarely exceeded 25% before the routine use of neoadjuvant chemotherapy and radiation [2]. The hallmark CROSS trial from 2012 established the benefit of neoadjuvant chemoradiation in patients with locally advanced disease [3]. Randomising 366 patients into either neoadjuvant carboplatin/paclitaxel followed by radiation versus upfront surgery in patients with T1N1M0 or T2-3 N(any) esophageal cancer. They found a significantly improved rate of negative resection margins (92% vs 69%) as well as a median survival benefit (49 months vs 24 months) with neoadjuvant therapy. Follow up reports from their cohort have since confirmed a sustained survival benefit of neoadjuvant therapy over upfront surgery [4, 5].
Currently, there is a lack of contemporary data on post-recurrence survival in patients after esophagectomy for esophageal cancer. Considering the growing use of adjuvant therapy after resection, identifying those at risk for disease recurrence could help guide decisions towards adjuvant treatment after surgery. The aim of this study was to distinguish factors associated with recurrence and survival after esophagectomy for esophageal carcinoma.
2. Materials and Methods
Data was retrospectively collected on all consecutive patients that underwent an esophagectomy for adeno- or squamous cell carcinoma of the esophagus between January 1st 2007, and May 31st, 2019 at a single institution. This study was conducted with approval from our institutional review board with a waiver of informed consent. All patients that underwent esophagectomy for esophageal carcinoma were included in the study. Patients with benign disease were excluded. The primary objective was to determine rate of recurrence and survival after curative intent esophagectomy. Secondary objectives included determining patterns of recurrence and distinguishing factors associated with recurrence and survival after esophagectomy for esophageal cancer.
2.1. Treatment and Definitions
All patients had esophageal cancer without distant metastases. Clinical stage was determined with computed tomography (CT) imaging with endoscopic ultrasound and/or positron emission tomography CT as indicated. Patients with locally advanced disease on presentation (≥T2, ≥N1) underwent neoadjuvant chemotherapy with paclitaxel/carboplatin followed by radiation therapy per the CROSS trial [3]. Surgical approach was determined on a case-by-case basis based on tumor location, lymph node spread and response to neoadjuvant therapy. All cases were discussed at a multidisciplinary tumor board pre and post treatment to assess response and determine resectability. The esophagus was divided into thirds for analysis, based on tumor location; upper (15-25 cm), middle (25-30 cm) and lower (30-42 cm). Recurrence was stratified into locoregional, distant and mixed recurrence.
Three surgeons performed all esophagectomies, 4-8 weeks after completion of neoadjuvant chemoradiation when utilized. Surgical approaches included minimally invasive and open transhiatal, Ivor Lewis and McKeown esophagectomy. Surgical approach was determined on a case-by-case basis based on tumor location, lymph node spread and response to neoadjuvant therapy.
2.2. Data and Statistical Analysis
Data on demographics, tumor characteristics, operative details, pathology, disease recurrence, location and survival were collected. Survival analysis was performed using Kaplan Meier graphs and compared between groups with the log rank test. Chi-square and Fischer’s exact tests were used to evaluate pre-determined factors associated with recurrence. Factors of interest included tumor grade, HER2 status, margin positivity, lymph node ratio (>0.2), neoadjuvant therapy, surgical approach and 90-day readmission. Factors with a p-value <0.1 on univariate analysis were included in a multivariate model testing association with recurrence. Data was stored using REDCap [6]. Statistical software utilized was Stata, version 16.1 (StataCorp, College Station, TX, USA).
3. Results
3.1. Demographics and Treatment
The study included 226 patients with either adenocarcinoma (n=206, 91.2%) or squamous cell carcinoma (n=20, 8.8%). Patient demographics, disease characteristics and treatment are listed in (Table 1). The mean age was 64 (range: 31-87) and 190 (83.7%) were male. The most common tumor location was the lower third (n=204, 89.9%). Majority of patients (n=164, 72.6%) had locally advanced disease on presentation and received neoadjuvant chemotherapy and radiation. The most common surgical approach was transhiatal esophagectomy (n=155, 74.2%). Traditional chemotherapy was utilized in patients receiving adjuvant treatment in the form of 5-fluorouracil and/or oxaliplatin. Adjuvant chemotherapy was utilized in 28 (12.4%) patients, all of which had either positive surgical margins or locally advanced disease (T3 and/or N1-N2) on final pathology. Of the 28 patients who received adjuvant treatment, 22 had undergone neoadjuvant treatment before surgery. Due to incomplete charting, data on tumor location (n=5), surgical approach (n=17), clinical T/N stage (n=60) and tumor grade (n=79) were missing. Immunotherapy was not utilized in the adjuvant setting during the study period. Of those tested for HER2 mutation (n=92), fifteen patients (16.3%) were positive. Out of those 15, two received trastuzumab in the adjuvant setting.
TABLE 1:
Patient Demographics, disease characteristics and treatment.
|
Variable |
N |
|
Age
(median, IQR) |
64.5
(21.2) |
|
Sex
(Male) |
190
(83.7%) |
|
Race
(white/Caucasian) |
212
(93.8%) |
|
BMI
(median, IQR) |
26.6
(7.2) |
|
Tumor location |
|
|
Upper
Third (0-20cm) |
5
(2.2%) |
|
Middle
Third (21-30cm) |
12
(5.3%) |
|
Lower
Third (>30cm) |
204
(89.9%) |
|
Surgical approach |
|
|
Transhiatal |
155
(74.2%) |
|
Ivor
Lewis |
24
(11.5%) |
|
Three-field |
30
(13.4%) |
|
T Stage |
|
|
Tis |
4
(2.5%) |
|
T1 |
29
(18.0%) |
|
T2 |
50
(31.1%) |
|
T3 |
78
(48.4%) |
|
T4 |
0
(0%) |
|
N Stage |
|
|
N0 |
119
(59.5%) |
|
N1 |
68
(34.0%) |
|
N2 |
10
(5.0%) |
|
N3 |
3
(1.5%) |
|
Tumor grade |
|
|
Well
differentiated |
22
(14.9%) |
|
Moderately
differentiated |
65
(44.2%) |
|
Poorly
differentiated |
60
(40.8%) |
|
Margin
positivity (n=212) |
18
(8.5%) |
|
HER2
positive (n=92) |
15
(16.3%) |
IQR: Interquartile range.
3.2. Recurrence and Survival
Recurrence was observed in 76 (33.8%) patients: 34 locoregional (44.7%), 27 distant (35.5%) and 15 (19.7%) mixed. Distant recurrence sites included liver (n=22, 28.9%) and lung (n=20, 26.3%) followed by bone (n=12, 15.8%), peritoneal (n=7, 9.2%) and other sites (n=16, 21.1%). Other sites included brain and various intra-abdominal sites. Median time to recurrence was 2.4 years (95% Confidence interval, 1.9-4.4). During a median 3.5 year follow up (range: 0.8-5.3), median overall survival was 3.6 years (95% CI, 3.0-5.8). Of the 76 with recurrence, 56 (73.7%) underwent neoadjuvant chemoradiation. The 5-year survival was 52.9% and 5-year disease-free survival was 46.4%. The median overall survival for those without recurrence was 9.2 years compared to 1.8 years for those who did experience recurrence (p<0.0001). Table 2 depicts post-recurrence survival by recurrence site. Post-recurrence survival was longer for those with locoregional compared to distal and mixed recurrence (7.8 vs 5.5 vs 5.2 months, p=0.59), although not to a significant extent (Figure 1).
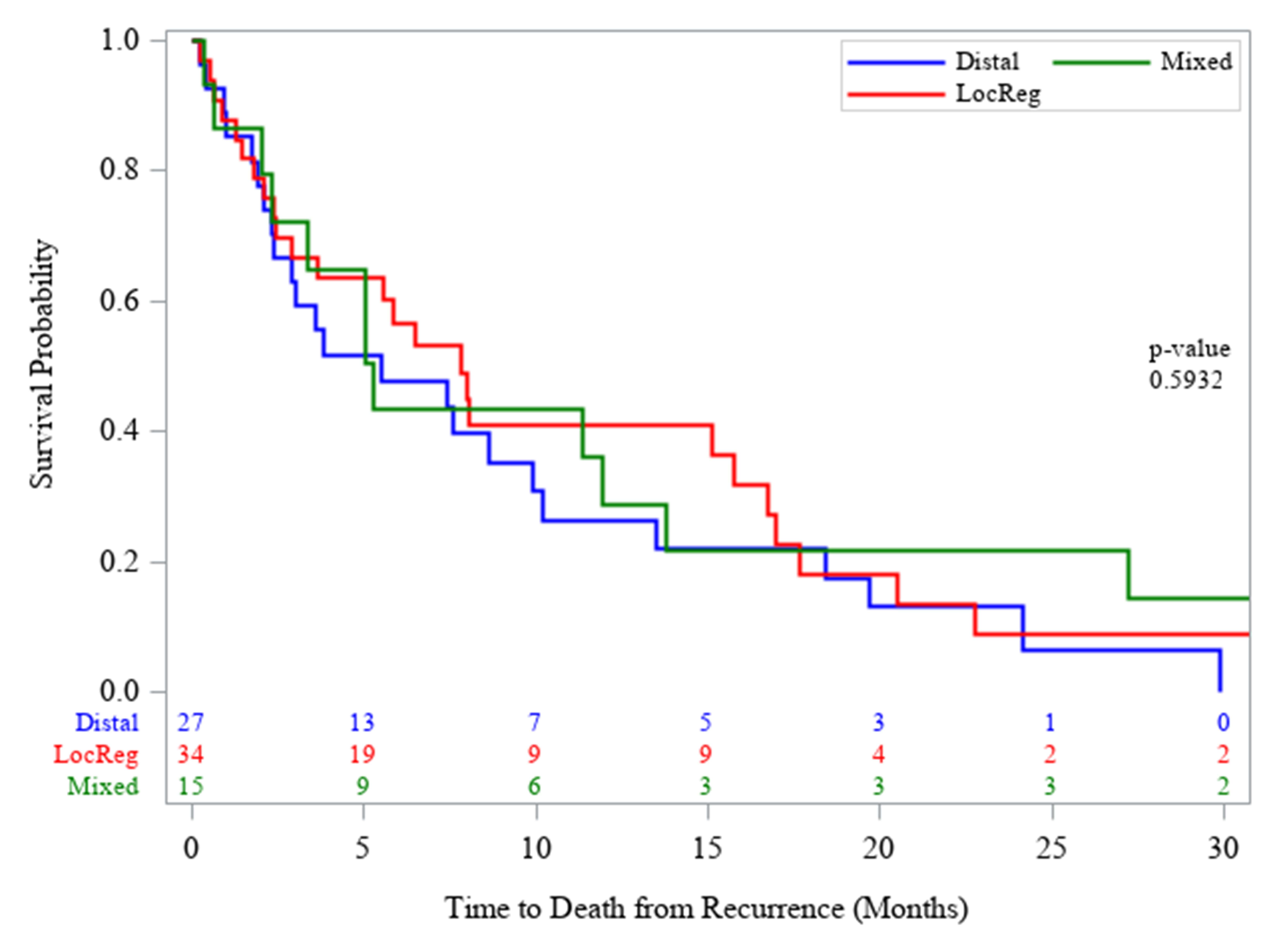
TABLE 2: Recurrence sites and post-recurrence survival
(n=76).
|
Site |
N (%) |
Median Survival (months) |
|
Locoregional |
34 (44.7) |
7.8 (95% CI: 2.9 - 15.7) |
|
Distant |
27 (35.5) |
5.5 (95% CI: 2.3 - 9.9) |
|
Mixed |
15 (19.7) |
5.3 (95% CI: 2.0 - 13.8) |
No difference in
post-recurrence survival was noted on log-rank test (p=0.59).
3.3. Factors Associated with Recurrence
Table 3 shows factors associated with recurrence. Of those tested, margin positivity, lymph node ratio (LNR) >0.2 and high tumor grade all correlated with recurrence (OR 5.4, 95% CI 1.8 - 14.8, OR 2.4, 95% CI 1.2 - 4.8 and OR 1.8, 95% CI 0.9 - 3.4, respectively) on univariate analysis. Additionally, recurrence was more likely to occur with higher T and N stages (p<0001 and p=0.003). Multivariate analysis included tumor grade, LNR >0.2, pathologic T- and N-stage, HER2- and margin status. Of those, only N1 status correlated with recurrence (OR 3.69, 95% CI: 1.44 - 9.47). Complete responders to neoadjuvant therapy (n=63) had a recurrence rate of 23.8% (n=15). Those with T1/Tis in their final pathology (n=65) had a recurrence rate of 16.9% (n=11). Of those 65 patients, 32 underwent upfront surgery of which 4 (12.5%) had disease recurrence.
TABLE 3: Factors associated with
recurrence.
|
Variable |
Recurrence |
Univariate analysis (OR (95% CI)) |
p-value |
Multivariate analysis (OR (95% CI)) |
P-value |
|
Tumor Grade (High) |
27 (34.6%) |
1.8 (0.9 - 3.4) |
0.049 |
|
0.814 |
|
Margin Positive |
13 (17.1%) |
5.4 (1.8 - 15.8) |
<0.001 |
|
0.089 |
|
Lymph Node Ratio (>0.2) |
21 (28.7%) |
2.4 (1.2 - 4.8) |
0.010 |
|
0.581 |
|
HER2 Positive |
8 (53.3%) |
2.8 (0.9 - 8.3) |
0.080 |
|
0.103 |
|
Neoadjuvant (Yes) |
58 (74.4) |
1.1 (0.6 - 2.1) |
0.718 |
|
-- |
|
Surgical Approach |
0.752 |
|
-- |
||
|
Transhiatal Esophagectomy -
Reference |
51 (32.9%) |
-- |
|
|
|
|
3-Field Esophagectomy
(McKeown) |
12 (40.0%) |
1.4 (0.6, 3.0) |
|
|
|
|
Ivor Lewis |
8 (33.3%) |
1.0 (0.4, 2.5) |
|
|
|
|
90 Day Readmission (Yes) |
29 (37.2%) |
1.4 (0.8 - 2.5) |
0.255 |
|
-- |
|
T-stage |
<0.001 |
|
0.680 |
||
|
CR - Reference |
15 (23.8%) |
-- |
|
|
|
|
T1/Tis |
11 (16.9%) |
0.6 (0.3, 1.6) |
|
|
|
|
T2 |
11 (36.7%) |
1.9 (0.7, 4.8) |
|
|
|
|
T3/T4 |
41 (61.2%) |
5.0 (2.4, 10.8) |
|
|
|
|
N-stage |
0.003 |
|
0.023 |
||
|
N0 - Reference |
43 (27.7%) |
-- |
|
-- |
|
|
N1 |
29 (52.7%) |
2.9 (1.5, 5.5) |
|
3.7 (1.4 - 9.5) |
|
|
N2/N3 |
6 (42.9%) |
2.0 (0.6, 6.0) |
|
2.1 (0.5 - 8.6) |
|
CR: Complete response.
*Transhiatal, Ivor Lewis, three-field.
4. Discussion
We sought to identify patterns of recurrence and post-recurrence survival after esophagectomy for patients with esophageal cancer. Our findings include a 33.8% overall rate of recurrence during a median 3.5 year follow up. Majority of all recurrences occurred at distant sites (55.2%). In 2015, a follow up report of the CROSS cohort analyzing disease recurrence was published [2]. Similar to our findings, the authors found the recurrence rate in those undergoing neoadjuvant therapy to be 34.7% and most recurrences were distant (75.4%). Of those with recurrence in our cohort, locoregional recurrence made up a higher proportion of all recurrences compared to the CROSS cohort, (44.7% versus 24.5%). This discrepancy could be due to fewer patients in our cohort receiving neoadjuvant chemoradiation (73.4%). Some patients in our cohort did not have locally advanced disease. Notably, the CROSS authors noted a drastic reduction in infield recurrence in their study.
Our aim was to determine survival stratified by recurrence site. Survival after locoregional recurrence was slightly better compared to those affected by systemic or mixed recurrence (7.8 vs 5.5 vs 5.3 months, p=0.59) in our cohort. This aggressive disease course following recurrence mimics those presenting with metastatic disease where overall survival has been described at 8-10 months [7]. Data is now emerging on the efficacy of nivolumab in the setting of advanced or recurrent esophageal cancer [8, 9]. Although mostly limited to squamous cell cancer, a modest survival benefit over chemotherapy alone has been described [9]. The poor post-recurrence survival, as seen in our study cohort, could serve as a prompt for further studies on adjuvant systemic therapy such as check-point inhibitors in patients with recurrence of esophageal adenocarcinoma.
Findings on univariate analysis indicate that those with positive resection margins, high grade tumors, and a LNR >0.2 are more likely to have disease recurrence. However, only nodal positivity remained significant on multivariate analysis (OR 3.69, 95% CI: 1.44 - 9.47). The majority of those who had locoregional recurrence (n=34) had undergone neoadjuvant chemoradiation (n=29). However, only 6 of those 34 patients underwent either two-field (Ivor Lewis) or three-field esophagectomy with the remainder undergoing transhiatal esophagectomy. Reports on the effects of surgical approach on lymph node yield have favored more aggressive mediastinal lymph node dissection. However, long-term survival differences have not been firmly established based on number of lymph nodes harvested during esophagectomy [10, 11]. Nonetheless, this would argue for the consideration of adjuvant therapy after esophagectomy, even in those patients with complete pathologic response to neoadjuvant therapy.
Margin positivity has been associated with worse outcomes in esophageal cancer [12-14]. A recent study utilizing data from the National Cancer Database investigated those with positive margins after resection without pretreatment [12]. They found 5-year survival to be 13.5%, noting a modest improvement in those who received adjuvant chemoradiation (16.9% vs 13.5%, p < 0.001). While most will respond to neoadjuvant chemoradiation as seen in the CROSS trial, there is a small subset of patients whose limited response puts them at risk for an incomplete resection. Adjuvant therapy should be considered in this cohort. LNR of >0.2 was chosen as the cutoff for our recurrence analysis. While LNR has been found to correlate with worse outcomes in multiple studies, 0.2 has commonly been cited as the median of positive nodes to total nodal yield [15-17]. Some authors have found the cutoff for LNR associated with improved response to adjuvant therapy to be 0.12, indicating that benefit of further treatment starts at an even lower ratio [16]. However, it must be noted that the bulk of the data on LNR comes from patients undergoing upfront resection. Herein, we show that LNR can be prognostic in pre-treated patients and could be considered when evaluating risk for recurrence.
Our data shows complete responders to neoadjuvant chemotherapy (n=63) had a recurrence of 23.8%. A notably higher recurrence rate than those with Tis or T1 on final pathology who underwent upfront resection (12.5%). This is similar to recent retrospective findings, where those undergoing surgery after complete response to neoadjuvant chemoradiation were found to have 26% rate of recurrence [18]. Although favorable response to neoadjuvant treatment portents a better long-term prognosis, these findings underscore the importance of vigilant post-operative surveillance. Additionally, the role of adjuvant treatment in this subgroup of patients could be explored. None of the complete responders in our cohort received adjuvant treatment after surgery.
The focus of recent studies on improving survival in patients with resectable esophageal cancer has centered around adjuvant therapy. A 2022 meta-analysis found those receiving adjuvant therapy after undergoing neoadjuvant treatment and surgery to have a 48% decrease in mortality at one year (risk ratio (RR) 0.51, 95% CI 0.41-065) [19]. In particular, they noted those with a positive nodal status and T3/T4 tumors benefitting most from adjuvant therapy (RR 0.61 and 0.51, respectively). This correlates with our findings indicating higher pathologic T and N stage associated with recurrence. The multi-center CheckMate 577 trial established a significant disease free survival benefit for R0 patients undergoing adjuvant nivolumab (22.4 months versus 11.0 months, p<0.001) after neoadjuvant treatment and surgery [20]. The data herein aids in identifying patients where higher index of suspicion for recurrence should be upheld. As the paradigm shifts towards more utilization of adjuvant therapy, identifying those who stand to benefit the most is warranted.
The limitations of this study include its retrospective design, subjecting it to missing or incorrect data. In contrast to some of the prior studies on this subject, our cohort includes patients with all stages of esophageal cancer, thus a considerable portion underwent surgery first. Hence, these results represent a valuable estimate of the risk, and patterns of recurrence in all comers undergoing esophagectomy for esophageal cancer.
5. Conclusion
We have shown that locally advanced esophageal cancer has a high recurrence rate, despite neoadjuvant chemoradiation. Furthermore, majority of recurrences involve distant sites. In our cohort, survival did not differ between those with locoregional and mixed recurrence. Those at risk are patients with higher T and N stage on pathology, positive tumor margins, high grade tumors and high lymph node ratio (>0.2). These data could aid in selecting patients for adjuvant treatment after curative esophageal cancer resection.
Acknowledgements
We would like to thank River Gibbs, MS and Jessica Parker, MS for assistance with data analysis and interpretation.
Conflicts of Interest
None.
Data Material
This material was presented as an oral presentation at the American College of Surgeons, Clinical Congress, virtual meeting, October 2021.
REFERENCES
[1] Freddie
Bray, Jacques Ferlay, Isabelle Soerjomataram, et al. “Global cancer statistics
2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in
185 countries.” CA Cancer J Clin, vol. 68, no. 6, pp. 394-424, 2018.
View at: Publisher
Site | PubMed
[2] Vera
Oppedijk, Ate van der Gaast, Jan J B van Lanschot, et al. “Patterns of
recurrence after surgery alone versus preoperative chemoradiotherapy and
surgery in the CROSS trials.” J Clin Oncol, vol. 32, no. 5, pp. 385-391,
2014. View at: Publisher
Site | PubMed
[3] P van
Hagen, M C C M Hulshof, J J B van Lanschot, et al. “Preoperative
Chemoradiotherapy for Esophageal or Junctional Cancer.” N Engl J Med vol. 366,
no. 22, pp. 2074-2084, 2012. View at: Publisher Site | PubMed
[4] Joel
Shapiro, J Jan B van Lanschot, Maarten C C M Hulshof, et al. “Neoadjuvant
chemoradiotherapy plus surgery versus surgery alone for oesophageal or
junctional cancer (CROSS): long-term results of a randomised controlled trial.”
Lancet Oncol, vol. 16, no. 9, pp. 1090-1098, 2015. View at: Publisher Site | PubMed
[5] Ben M
Eyck, J Jan B van Lanschot, Maarten C C M Hulshof, et al. “Ten-Year Outcome of
Neoadjuvant Chemoradiotherapy Plus Surgery for Esophageal Cancer: The
Randomized Controlled CROSS Trial.” J Clin Oncol, vol. 39, no. 18 pp.
1995-2004, 2021. View at: Publisher Site | PubMed
[6] Paul A
Harris, Robert Taylor, Robert Thielke, et al. “Research electronic data capture
(REDCap)-A metadata-driven methodology and workflow process for providing
translational research informatics support.” J Biomed Inform, vol. 42,
no. 2, pp. 377-381, 2009. View at: Publisher Site | PubMed
[7] William
P Tew, David P Kelsen, David H Ilson “Targeted Therapies for Esophageal
Cancer.” Oncologist, vol. 10, no. 8, pp. 590-601, 2005. View at: Publisher Site | PubMed
[8] Takashi
Kojima , Hiroki Hara , Kensei Yamaguchi, et al. “Phase II study of nivolumab
(ONO-4538/BMS-936558) in patients with esophageal cancer: Preliminary report of
overall survival.” Journal of Clinical Oncology, vol. 34, no. 4, pp.
TPS175, 2016. View at: Publisher Site
[9] Yuichiro
Doki 1, Jaffer A Ajani 1, Ken Kato, et al. “Nivolumab Combination Therapy in Advanced
Esophageal Squamous-Cell Carcinoma.” N Engl J Med, vol. 386, no. 5, pp.
449-462, 2022. View at: Publisher
Site | PubMed
[10] C S
Wolff, S F Castillo, D R Larson, et al., “Ivor Lewis approach is superior to
transhiatal approach in retrieval of lymph nodes at esophagectomy.” Dis
Esophagus, vol. 21, no. 4, pp. 328-333, 2008. View at: Publisher Site | PubMed
[11] Nirmal K
Veeramachaneni, Jennifer B Zoole, Paul A Decker, et al. “Lymph Node Analysis in
Esophageal Resection: American College of Surgeons Oncology Group Z0060 Trial.”
Ann Thorac Surg, vol. 86, no. 2, pp. 418-421, 2008. View at: Publisher Site | PubMed
[12] Jeffrey
Javidfar, Paul J Speicher, Matthew G Hartwig, et al. “Impact of Positive
Margins on Survival in Patients Undergoing Esophagogastrectomy for Esophageal
Cancer.” Ann Thorac Surg, vol. 101, no. 3, pp. 1060-1067, 2016. View at:
Publisher Site | PubMed
[13] Shao-Hua
Xie, Giola Santoni, Kalle Mälberg, et al. “Prediction Model of Long-term
Survival After Esophageal Cancer Surgery.” Ann Surg, vol, 273, no. 5,
pp. 933-939, 2019. View at: Publisher Site | PubMed
[14] Grace J
Kim, Matthew Koshy, Alexandra L Hanlon, et al. “The Benefit of Chemotherapy in
Esophageal Cancer Patients With Residual Disease After Trimodality Therapy.” Am
J Clin Oncol, vol. 39, no. 2, pp. 136-141, 2016. View at: Publisher Site | PubMed
[15] Castigliano
M Bhamidipati, George J Stukenborg, Christopher J Thomas, et al. “Pathologic
Lymph Node Ratio Is a Predictor of Survival in Esophageal Cancer.” Ann
Thorac Surg vol. 94, no. 5, pp. 1643-1651, 2012. View at: Publisher Site | PubMed
[16] Vignesh
Raman, Oliver K Jawitz, Norma E Farrow, et al. “The Relationship Between Lymph
Node Ratio and Survival Benefit With Adjuvant Chemotherapy in Node-positive
Esophageal Adenocarcinoma.” Ann Surg, vol. 275, no. 3, pp. e562-e567,
2022. View at: Publisher Site | PubMed
[17] Hiroshi
Miyata, Koji Tanaka, Tomoki Makino, et al. “The Impact of Pathological Tumor
Regression and Nodal Status on Survival and Systemic Disease in Patients
Undergoing Neoadjuvant Chemotherapy for Esophageal Squamous Cell Carcinoma.” Ann
Surg Oncol, vol. 25, no. 8, pp. 2409-2417, 2018. View at: Publisher Site | PubMed
[18] Arianna Barbetta,
Smita Sihag, Tamar Nobel, et al. “Patterns and risk of recurrence in patients
with esophageal cancer with a pathologic complete response after
chemoradiotherapy followed by surgery.” J Thorac Cardiovasc Surg, vol.
157, no. 3, pp. 1249-1259.e5, 2019. View at: Publisher Site | PubMed
[19] Yung Lee, Yasith Samarasinghe, Michael H Lee, et al. “Role of Adjuvant Therapy in Esophageal Cancer Patients After Neoadjuvant Therapy and Esophagectomy: A Systematic Review and Meta-analysis.” Ann Surg, vol. 275, no. 1, pp. 91-98, 2022. View at: Publisher Site | PubMed
[20] Ronan J Kelly, Jaffer A Ajani, Jaroslaw Kuzdzal, et al. “Adjuvant Nivolumab in Resected Esophageal or Gastroesophageal Junction Cancer.” N Engl J Med, vol. 384, no. 13, pp. 1191-1203, 2021. View at: Publisher Site | PubMed
